Embark on a journey of chemical exploration with Unit 2 Review Chemistry Answers, your ultimate guide to unraveling the complexities of chemistry. From the core principles of chemical reactions to the fascinating world of thermochemistry, this comprehensive resource will illuminate your path to success in chemistry.
Delve into the intricate world of gases and their properties, unravel the secrets of solutions and solubility, and master the concepts of acids, bases, and pH. Our team of chemistry experts has meticulously crafted this guide to provide you with a deep understanding of the fundamental concepts that shape the world of chemistry.
Chemistry Unit 2 Review Answers: Key Concepts
Unit 2 of the chemistry curriculum introduces fundamental principles and theories that provide a solid foundation for understanding chemical reactions and their applications. It covers essential concepts that lay the groundwork for further exploration in the field of chemistry.
This unit focuses on the structure of atoms, the periodic table, and chemical bonding. These concepts are crucial for comprehending the behavior and properties of elements and compounds.
Atomic Structure
- Describes the fundamental particles of atoms: protons, neutrons, and electrons.
- Explains the concept of atomic number and mass number.
- Introduces the electron configuration and its significance in determining chemical properties.
Periodic Table
- Explores the organization of elements in the periodic table.
- Highlights the periodic trends in properties such as atomic radius, ionization energy, and electronegativity.
- Emphasizes the relationship between an element’s position in the periodic table and its chemical reactivity.
Chemical Bonding
- Introduces the different types of chemical bonds: ionic, covalent, and metallic.
- Explains the formation and properties of each type of bond.
- Discusses the role of electronegativity in determining the type of bond formed.
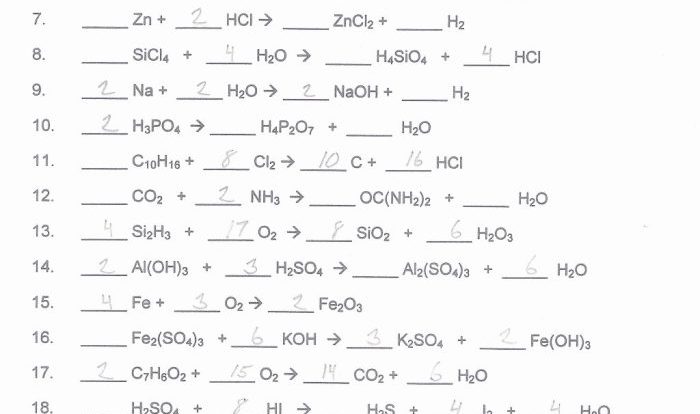
Reactions and Stoichiometry
Chemical reactions are the processes in which substances undergo chemical transformations to form new substances. They are crucial in chemistry, as they allow us to understand how elements and compounds interact, create new materials, and comprehend the changes occurring in our surroundings.Chemical
reactions can be classified into various types based on the changes they bring about. Combustion reactions involve the reaction of a substance with oxygen, releasing energy in the form of heat and light. Synthesis reactions combine two or more substances to form a more complex product.
Decomposition reactions break down a compound into simpler substances. Displacement reactions occur when one element replaces another in a compound.Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. By balancing chemical equations, we can determine the exact proportions of reactants required to produce a given amount of products.
Stoichiometry helps us predict the outcome of reactions, optimize chemical processes, and understand the efficiency of reactions.
Combustion Reactions
Combustion reactions are characterized by the rapid reaction of a substance with oxygen, releasing heat and light. Examples include burning wood, propane in a gas stove, or the reaction of magnesium with oxygen to form magnesium oxide.
Synthesis Reactions
Synthesis reactions involve the combination of two or more substances to form a more complex product. Examples include the reaction of hydrogen and oxygen to form water, the synthesis of sodium chloride from sodium and chlorine, or the formation of carbon dioxide from carbon and oxygen.
Decomposition Reactions
Decomposition reactions are the opposite of synthesis reactions, where a single compound breaks down into simpler substances. Examples include the decomposition of water into hydrogen and oxygen, the breakdown of calcium carbonate into calcium oxide and carbon dioxide, or the dissociation of hydrogen peroxide into water and oxygen.
Displacement Reactions
Displacement reactions occur when one element replaces another in a compound. Examples include the reaction of iron with copper sulfate to form iron sulfate and copper, or the displacement of hydrogen from water by sodium to form sodium hydroxide and hydrogen gas.
Gases and Their Properties
Gases are a unique state of matter characterized by their ability to expand and fill the volume of their container. Understanding the behavior of gases is crucial in various fields of science, including chemistry, physics, and engineering.The properties of gases are governed by three fundamental variables: pressure, volume, and temperature.
These variables are interconnected and can be described by the ideal gas law, which provides a mathematical relationship between them.
Pressure
Pressure is the force exerted by a gas per unit area. It is measured in units of pascals (Pa) or atmospheres (atm). Pressure can be increased by either increasing the number of gas molecules in a given volume or by decreasing the volume while keeping the number of molecules constant.
Volume
Volume is the amount of space occupied by a gas. It is measured in units of liters (L) or cubic meters (m^3). Volume can be changed by altering the size of the container or by changing the pressure while keeping the temperature constant.
Temperature
Temperature is a measure of the average kinetic energy of the gas molecules. It is measured in units of Kelvin (K) or degrees Celsius (°C). Increasing the temperature of a gas causes the molecules to move faster, which increases the pressure and volume while keeping the number of molecules constant.
Ideal Gas Law, Unit 2 review chemistry answers
The ideal gas law is a mathematical equation that relates pressure, volume, and temperature for an ideal gas. It is expressed as:PV = nRTwhere:* P is the pressure in pascals (Pa)
- V is the volume in cubic meters (m^3)
- n is the number of moles of gas
- R is the ideal gas constant (8.31 J/mol·K)
- T is the temperature in Kelvin (K)
The ideal gas law is a powerful tool for predicting the behavior of gases under different conditions. It has numerous applications in chemistry, including determining the molar mass of gases, calculating gas densities, and predicting the behavior of gases in reactions.
Gas Laws
In addition to the ideal gas law, there are several other gas laws that describe the behavior of gases under specific conditions. These laws include:* Boyle’s Law: Describes the relationship between pressure and volume at constant temperature.
Charles’s Law
Describes the relationship between volume and temperature at constant pressure.
Gay-Lussac’s Law
Describes the relationship between pressure and temperature at constant volume.
Avogadro’s Law
Describes the relationship between volume and the number of moles of gas at constant temperature and pressure.These gas laws are essential for understanding the behavior of gases in various applications, such as gas chromatography, pressure cookers, and weather forecasting.
Solutions and Solubility
Solutions are homogeneous mixtures of two or more chemical substances. The substance present in the largest amount is called the solvent, while the substance present in the smaller amount is called the solute. Solutions can be classified into two types: homogeneous and heterogeneous.
Homogeneous Solutions
Homogeneous solutions are those in which the solute is evenly distributed throughout the solvent. This means that the solution has the same composition at all points. Examples of homogeneous solutions include saltwater, sugar water, and air.
Heterogeneous Solutions
Heterogeneous solutions are those in which the solute is not evenly distributed throughout the solvent. This means that the solution has different compositions at different points. Examples of heterogeneous solutions include sand in water, oil in water, and salad dressing.
Factors Affecting Solubility
The solubility of a substance is the maximum amount of that substance that can be dissolved in a given amount of solvent at a given temperature. The solubility of a substance is affected by several factors, including:
- Temperature:The solubility of most solids and liquids increases with increasing temperature. This is because the higher the temperature, the more energy the solvent molecules have, and the more solute molecules they can dissolve.
- Pressure:The solubility of gases in liquids increases with increasing pressure. This is because the higher the pressure, the more gas molecules are forced into the liquid.
- Nature of the solvent and solute:The solubility of a substance is also affected by the nature of the solvent and solute. In general, polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes.
Solubility Calculations
The solubility of a substance can be calculated using the following equation:
“`Solubility = (Mass of solute / Volume of solution) x 100%“`
For example, if 10 g of salt is dissolved in 100 mL of water, the solubility of the salt is:
“`Solubility = (10 g / 100 mL) x 100% = 10%“`
Acids, Bases, and pH
Acids and bases are two important classes of chemical compounds that play a crucial role in various chemical processes. Acids are substances that donate protons (H+ ions), while bases are substances that accept protons.Acids and bases exhibit distinct properties. Acids are typically sour to taste, corrosive to skin, and turn blue litmus paper red.
Bases, on the other hand, are bitter to taste, slippery to touch, and turn red litmus paper blue. The strength of an acid or base is measured by its pH value. pH is a measure of the concentration of hydrogen ions in a solution.
A pH of 7 is neutral, while a pH below 7 indicates an acidic solution and a pH above 7 indicates a basic solution.Acid-base reactions are chemical reactions that involve the transfer of protons from an acid to a base.
These reactions are important in many chemical processes, such as the neutralization of acids and bases, the formation of salts, and the regulation of pH in biological systems.
Chemical Bonding
Chemical bonding is the process by which atoms, ions, or molecules are held together by attractive forces. The strength of these forces determines the physical and chemical properties of the resulting substance. There are three main types of chemical bonds: ionic, covalent, and metallic.
Ionic Bonding
Ionic bonding is the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). This type of bond is formed when one or more electrons are transferred from one atom to another. Ionic bonds are typically strong and result in the formation of ionic compounds, such as sodium chloride (NaCl) and potassium iodide (KI).
Covalent Bonding
Covalent bonding is the sharing of electrons between two or more atoms. This type of bond is formed when atoms have unpaired electrons in their valence shells. Covalent bonds are typically weaker than ionic bonds and result in the formation of molecular compounds, such as water (H 2O) and carbon dioxide (CO 2).
Metallic Bonding
Metallic bonding is the attraction between positively charged metal ions and a sea of mobile electrons. This type of bond is formed when metal atoms lose one or more electrons to form positively charged ions. Metallic bonds are typically strong and result in the formation of metals, such as iron, copper, and aluminum.
Thermochemistry: Unit 2 Review Chemistry Answers
Thermochemistry is the branch of chemistry that studies the energy changes associated with chemical reactions. It helps us understand how energy is absorbed or released during chemical reactions and how these changes affect the properties of substances.
Thermochemistry has various applications in chemistry, including:
- Predicting the spontaneity of reactions
- Designing new materials with desired properties
- Understanding and controlling chemical processes in industrial settings
Laws of Thermodynamics
The laws of thermodynamics are fundamental principles that govern energy transformations in chemical reactions. These laws include:
- First law of thermodynamics:Energy cannot be created or destroyed, only transferred or transformed.
- Second law of thermodynamics:The entropy of an isolated system always increases over time.
- Third law of thermodynamics:The entropy of a perfect crystal at absolute zero is zero.
Exothermic and Endothermic Reactions
Chemical reactions can be classified as exothermic or endothermic based on whether they release or absorb energy, respectively.
- Exothermic reactions:Release energy into the surroundings, causing an increase in temperature. Example: Combustion of methane.
- Endothermic reactions:Absorb energy from the surroundings, causing a decrease in temperature. Example: Dissolution of ammonium chloride in water.
The enthalpy change (ΔH) of a reaction is a measure of the heat absorbed or released during the reaction. A negative ΔH indicates an exothermic reaction, while a positive ΔH indicates an endothermic reaction.
Frequently Asked Questions
What is the significance of stoichiometry in chemistry?
Stoichiometry is crucial in chemistry as it allows us to determine the exact quantitative relationships between reactants and products in a chemical reaction. By understanding stoichiometry, we can predict the amount of reactants and products involved in a reaction, ensuring efficient and accurate experimentation.
How does temperature affect the behavior of gases?
Temperature plays a significant role in the behavior of gases. According to the ideal gas law, the volume of a gas is directly proportional to its temperature. As temperature increases, the average kinetic energy of gas molecules increases, causing them to move faster and occupy a larger volume.
What factors influence the solubility of substances?
Several factors influence the solubility of substances, including temperature, pressure, and the nature of the solute and solvent. Temperature generally has a positive effect on solubility, while pressure has a more significant impact on gases than solids or liquids. The polarity and intermolecular forces between the solute and solvent also play a crucial role in determining solubility.