Chapter 7 worksheet #1 balancing chemical equations – Chapter 7 Worksheet #1: Balancing Chemical Equations provides a comprehensive overview of the fundamental principles and techniques involved in balancing chemical equations. This worksheet serves as an essential tool for students to develop a strong understanding of stoichiometry and the conservation of mass in chemical reactions.
Through a structured and engaging approach, this worksheet guides students through the step-by-step process of balancing chemical equations. It begins with a clear definition of balancing chemical equations and the significance of stoichiometry. The worksheet then delves into the practical steps involved in balancing equations, including the adjustment of coefficients and the use of mole ratios.
1. Introduction to Balancing Chemical Equations
Balancing chemical equations involves adjusting the coefficients in front of each chemical formula to ensure that the number of atoms of each element on the reactants’ side equals the number of atoms of that element on the products’ side. This is crucial because chemical reactions must adhere to the law of conservation of mass, which states that matter cannot be created or destroyed during a reaction.
The concept of stoichiometry, which deals with the quantitative relationships between reactants and products in chemical reactions, is closely linked to balancing equations. Mole ratios, which represent the relative number of moles of reactants and products, play a significant role in determining the coefficients in a balanced equation.
2. Steps for Balancing Chemical Equations
Balancing chemical equations involves a step-by-step process:
- Write the unbalanced equation, including all reactants and products.
- Start by balancing the most complex compound or the compound with the highest number of atoms.
- Balance the atoms one element at a time, adjusting the coefficients in front of each chemical formula.
- Check the balance of all atoms to ensure that the equation is balanced.
Adjusting coefficients should be done carefully to avoid altering the chemical formulas or creating new compounds.
3. Balancing Simple Equations
For simple equations involving 2-3 reactants and products, balancing can be done by trial and error or by using algebraic methods. For example, the unbalanced equation:
Fe + HCl → FeCl 2+ H 2
can be balanced by adjusting the coefficients as follows:
Fe + 2HCl → FeCl 2+ H 2
Here, the coefficients are adjusted to ensure that there are two atoms of hydrogen on both sides of the equation.
4. Balancing Complex Equations: Chapter 7 Worksheet #1 Balancing Chemical Equations
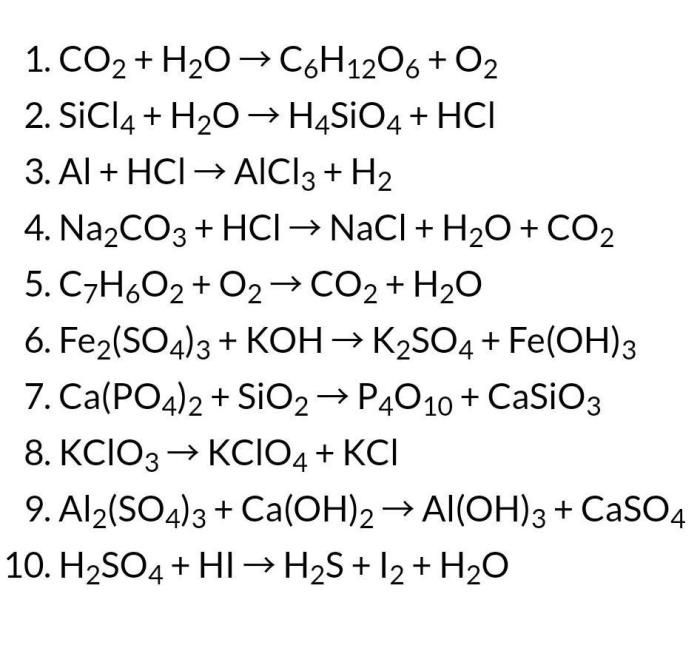
For more complex equations with multiple reactants and products, balancing can be more challenging. Strategies such as:
- Half-reaction method
- Oxidation-reduction method
- Matrix method
can be employed to determine the appropriate coefficients. These methods involve breaking down the equation into smaller, more manageable parts and then combining the balanced half-reactions or using algebraic equations to solve for the coefficients.
5. Practice Problems
To test understanding, students can practice balancing equations of varying difficulty levels. These problems can range from simple equations with 2-3 reactants and products to more complex equations involving multiple compounds and redox reactions.
6. Advanced Techniques
In certain cases, advanced techniques may be necessary to balance equations. These techniques include:
- Balancing equations in solution
- Balancing equations with fractional coefficients
- Balancing equations involving gases
These techniques require a deeper understanding of chemical principles and are typically encountered in more advanced chemistry courses.
7. Applications of Balancing Chemical Equations
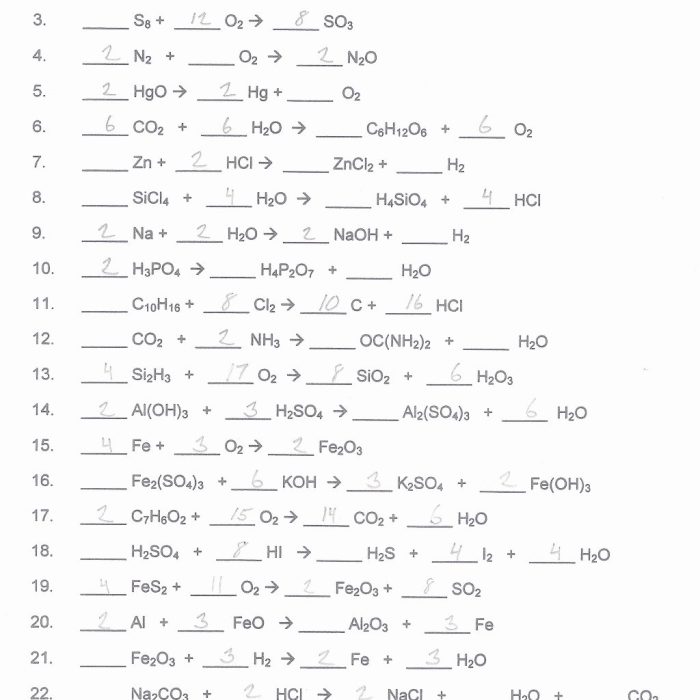
Balancing chemical equations has practical applications in various fields, including:
- Stoichiometric calculations: Determining the quantitative relationships between reactants and products in chemical reactions.
- Chemical reactions: Predicting the products and their relative amounts in a given reaction.
- Chemical synthesis: Designing and optimizing chemical reactions for the production of desired compounds.
Balanced equations provide a foundation for understanding and manipulating chemical reactions, making them essential in various scientific and industrial applications.
Questions and Answers
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side of the equation matches the number of atoms of the same element on the products’ side. This reflects the law of conservation of mass, which states that mass cannot be created or destroyed in a chemical reaction.
What is the mole ratio?
The mole ratio is the ratio of the number of moles of two substances involved in a chemical reaction. It is used to determine the stoichiometric proportions of reactants and products in a balanced chemical equation.
What are the steps involved in balancing chemical equations?
Balancing chemical equations typically involves the following steps: identifying the unbalanced equation, adjusting coefficients to balance the number of atoms of each element, and checking the final equation to ensure it is balanced.


