Introductory Chemistry Tro 6th Edition embarks on a captivating journey into the realm of chemistry, laying a solid foundation for students seeking to unravel the intricacies of matter, energy, and chemical reactions. Delving into the periodic table’s organizational principles, this comprehensive guide elucidates the fundamental concepts of chemical bonding and molecular structure, setting the stage for a deeper understanding of the chemical world.
With a focus on clarity and precision, Introductory Chemistry Tro 6th Edition empowers readers to navigate the complexities of stoichiometry and quantitative relationships, unraveling the mysteries of mole calculations and chemical equation balancing. The text masterfully presents the diverse types of chemical reactions and their distinctive characteristics, equipping students with a comprehensive understanding of chemical transformations.
Key Concepts and Theories in Introductory Chemistry

Chemistry is the study of matter and its properties, as well as the changes it undergoes. Matter is anything that has mass and occupies space. Energy is the ability to do work. Chemical reactions are processes that involve the rearrangement of atoms and molecules.
The Periodic Table
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number, electron configuration, and recurring chemical properties. It is a useful tool for organizing and understanding the elements.
Chemical Bonding
Chemical bonding is the attraction between atoms that holds them together to form molecules and compounds. There are three main types of chemical bonds: ionic bonds, covalent bonds, and metallic bonds.
Molecular Structure
Molecular structure refers to the arrangement of atoms within a molecule. It is determined by the types of chemical bonds present and the number and arrangement of atoms in the molecule.
Stoichiometry and Quantitative Relationships: Introductory Chemistry Tro 6th Edition

Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It is used to determine the amount of reactants and products that are involved in a reaction.
Mole Calculations
Mole calculations are used to convert between the mass of a substance and the number of moles of that substance. The mole is the SI unit of amount of substance.
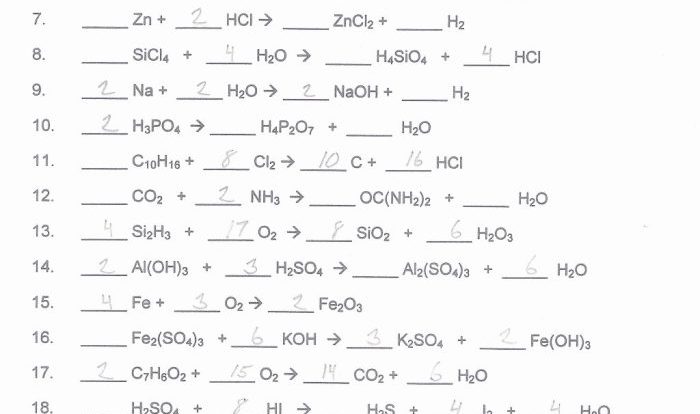
Balancing Chemical Equations
Balancing chemical equations is essential for stoichiometry. A balanced chemical equation shows the number of moles of each reactant and product involved in the reaction.
Types of Chemical Reactions, Introductory chemistry tro 6th edition
There are many different types of chemical reactions, each with its own characteristics. Some common types of reactions include:
- Combination reactions
- Decomposition reactions
- Single-replacement reactions
- Double-replacement reactions
States of Matter and Solutions
Matter can exist in three states: solid, liquid, and gas. Each state has its own unique properties.
Solubility
Solubility is the ability of a substance to dissolve in a solvent. It is affected by the temperature, pressure, and nature of the substance and solvent.
Concentration
Concentration is a measure of the amount of solute in a solution. It can be expressed in units of molarity, molality, or percent composition.
Colligative Properties
Colligative properties are properties of solutions that depend on the concentration of the solute, but not on the identity of the solute. Some common colligative properties include freezing point depression, boiling point elevation, and osmotic pressure.
Questions and Answers
What is the key focus of Introductory Chemistry Tro 6th Edition?
Introductory Chemistry Tro 6th Edition provides a comprehensive overview of the fundamental concepts of chemistry, including matter, energy, chemical reactions, stoichiometry, states of matter, thermodynamics, acids, bases, electrochemistry, organic chemistry, and biochemistry.
How does Introductory Chemistry Tro 6th Edition approach stoichiometry?
Introductory Chemistry Tro 6th Edition presents stoichiometry in a clear and systematic manner, emphasizing mole calculations, chemical equation balancing, and the determination of limiting reactants. It also explores the different types of chemical reactions and their characteristics.
What are the strengths of Introductory Chemistry Tro 6th Edition?
Introductory Chemistry Tro 6th Edition is renowned for its clarity, precision, and comprehensive coverage of chemistry fundamentals. It features numerous solved examples, practice exercises, and end-of-chapter questions to reinforce understanding.