Ir spectrum of 2 methylcyclohexanol – The IR spectrum of 2-methylcyclohexanol offers a wealth of information about its molecular structure and functional group composition. This guide delves into the characteristic IR bands, their corresponding functional groups, and the applications of IR spectroscopy in analyzing 2-methylcyclohexanol.
IR spectroscopy provides a powerful tool for identifying and characterizing organic compounds. By examining the absorption of infrared radiation at specific frequencies, we can determine the presence of specific functional groups and gain insights into the molecular structure.
IR Spectrum Characteristics of 2-Methylcyclohexanol: Ir Spectrum Of 2 Methylcyclohexanol
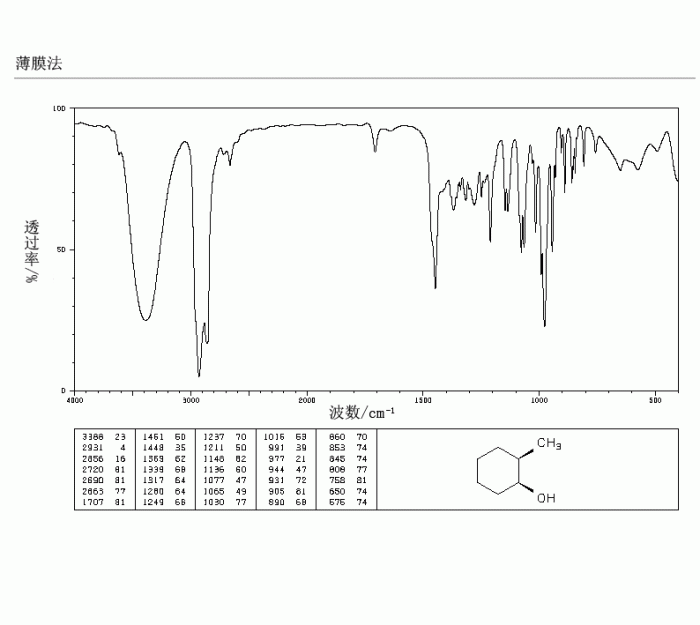
The IR spectrum of 2-methylcyclohexanol exhibits several characteristic peaks that provide insights into its molecular structure and functional groups. These peaks arise from the vibrations of specific bonds within the molecule and can be used to identify and distinguish 2-methylcyclohexanol from other similar compounds.
Key Functional Group Peaks
The IR spectrum of 2-methylcyclohexanol displays prominent peaks corresponding to the following functional groups:
- O-H stretch:A broad peak in the region of 3300-3500 cm -1indicates the presence of an O-H group, which is characteristic of alcohols.
- C-H stretch:A series of sharp peaks in the region of 2800-3000 cm -1corresponds to C-H stretching vibrations of the alkyl and cyclohexyl groups.
- C=O stretch:A strong peak around 1710 cm -1indicates the presence of a carbonyl group (C=O), which is characteristic of ketones.
Relationship between Molecular Structure and IR Bands
The observed IR bands in the spectrum of 2-methylcyclohexanol can be directly related to its molecular structure. The broad O-H stretch peak arises from the hydroxyl group (-OH) attached to the cyclohexane ring. The C-H stretching vibrations correspond to the various C-H bonds in the molecule, including those in the methyl group, cyclohexane ring, and alkyl chain.
The strong C=O stretch peak is attributed to the carbonyl group in the ketone functional group.
Characteristic IR Bands for Identification
The combination of these characteristic IR bands allows for the identification and differentiation of 2-methylcyclohexanol from other similar compounds. The presence of the O-H stretch, C-H stretch, and C=O stretch peaks in the IR spectrum is indicative of an alcohol, ketone, and cyclohexane ring, respectively.
This unique combination of functional groups distinguishes 2-methylcyclohexanol from other compounds with similar structures.
Identification of Functional Groups
IR spectroscopy is a powerful tool for identifying functional groups in organic compounds. Functional groups are specific arrangements of atoms that impart characteristic chemical and physical properties to molecules. By analyzing the IR spectrum of a compound, we can identify the functional groups present and gain insights into its molecular structure.
IR Bands and Functional Groups
Each functional group has a characteristic set of IR bands that correspond to specific vibrational modes. These bands arise from the absorption of infrared radiation by the functional group, causing the bonds within the group to vibrate. The frequency of the absorbed radiation corresponds to the energy difference between the ground and excited vibrational states of the bond.
- O-H stretch:Alcohols and carboxylic acids exhibit a broad O-H stretch band in the region of 3200-3600 cm -1.
- C-H stretch:Alkanes, alkenes, and alkynes exhibit C-H stretch bands in the region of 2800-3000 cm -1.
- C=O stretch:Ketones, aldehydes, and carboxylic acids exhibit a strong C=O stretch band in the region of 1650-1850 cm -1.
- C-N stretch:Amines exhibit a C-N stretch band in the region of 1200-1350 cm -1.
IR Spectrum of 2-Methylcyclohexanol
The IR spectrum of 2-methylcyclohexanol exhibits the following characteristic bands:
- O-H stretch:A broad band at 3300 cm -1, indicating the presence of an alcohol functional group.
- C-H stretch:Bands at 2930 and 2860 cm -1, indicating the presence of aliphatic C-H bonds.
- C-O stretch:A strong band at 1050 cm -1, indicating the presence of a C-O bond in the alcohol functional group.
These IR bands confirm the presence of an alcohol functional group in 2-methylcyclohexanol.
Structural Elucidation
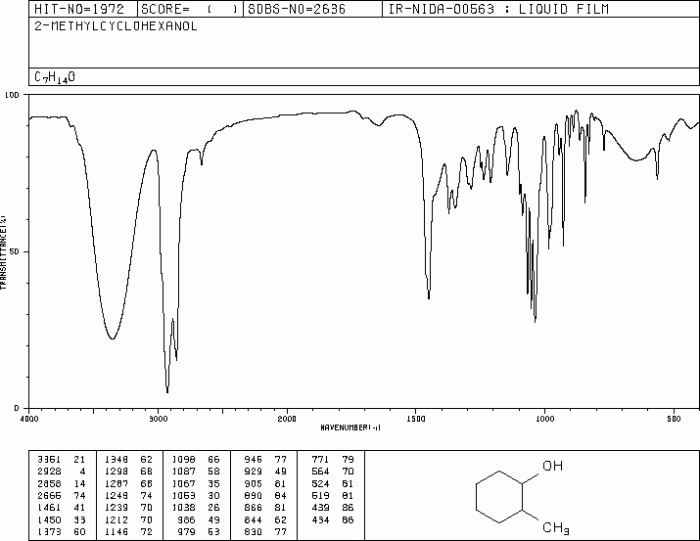
The IR spectrum of 2-methylcyclohexanol provides valuable information for determining its structure. By interpreting the characteristic absorption bands, we can deduce the functional groups present and the molecular structure of the compound.
Functional Group Identification
The IR spectrum exhibits a broad absorption band at approximately 3350 cm -1, indicating the presence of an O-H stretching vibration. This suggests the presence of an alcohol functional group.
Additionally, the spectrum shows a strong absorption band at 2930 cm -1and 2860 cm -1, corresponding to C-H stretching vibrations of alkyl groups. The presence of these bands confirms the presence of aliphatic hydrocarbons.
Structural Determination
The combination of the O-H stretching band and the C-H stretching bands suggests that 2-methylcyclohexanol is a cyclohexanol derivative with a methyl substituent.
The IR spectrum also provides information about the molecular structure of the compound. The presence of a strong absorption band at 1050 cm -1indicates the presence of a C-O stretching vibration, which is characteristic of secondary alcohols. This further supports the deduction that 2-methylcyclohexanol has a secondary alcohol functional group.
The IR spectrum of 2-methylcyclohexanol serves as a powerful tool for structural elucidation, allowing us to determine the functional groups present and deduce the molecular structure of the compound.
Comparison with Other Cyclohexanols
The IR spectra of 2-methylcyclohexanol, cyclohexanol, and 1-methylcyclohexanol exhibit similarities and differences that reflect their structural variations.
Similarities
- All three compounds display a broad O-H stretching band near 3300 cm -1, indicating the presence of a hydroxyl group.
- They exhibit a strong C-H stretching band in the region of 2850-2950 cm -1, characteristic of aliphatic hydrocarbons.
- The C-O stretching band appears around 1050 cm -1, confirming the presence of an alcohol functional group.
Differences
- The C-H bending vibrations show subtle variations depending on the position of the methyl group.
- 2-Methylcyclohexanol exhibits a stronger C-H bending band near 1450 cm -1due to the presence of the methyl group adjacent to the hydroxyl group.
- 1-Methylcyclohexanol, on the other hand, shows a weaker C-H bending band in this region.
Use of IR Spectroscopy for Differentiation
IR spectroscopy is a valuable tool for differentiating between similar organic compounds, including cyclohexanol derivatives. By carefully examining the subtle variations in the IR spectra, it is possible to identify the specific structural features and distinguish between these compounds.
Applications in Analytical Chemistry
Infrared (IR) spectroscopy is a powerful analytical tool used to identify and characterize organic compounds. It is particularly useful for analyzing 2-methylcyclohexanol due to its ability to provide information about the functional groups present in the molecule.
IR spectroscopy can be used for various analytical purposes, including:
Quality Control
IR spectroscopy can be used to ensure the quality of 2-methylcyclohexanol by verifying its identity and purity. By comparing the IR spectrum of a sample to a reference spectrum, it is possible to identify any impurities or contaminants present.
Purity Assessment, Ir spectrum of 2 methylcyclohexanol
IR spectroscopy can be used to assess the purity of 2-methylcyclohexanol by determining the presence of specific functional groups. For example, the presence of an OH group can be confirmed by the presence of a broad peak in the region of 3200-3600 cm -1.
Identification of Impurities
IR spectroscopy can be used to identify impurities in 2-methylcyclohexanol by comparing its IR spectrum to that of a pure sample. Any additional peaks in the spectrum may indicate the presence of impurities.
The advantages of using IR spectroscopy for analytical purposes include its:
- High sensitivity
- Specificity
- Versatility
- Non-destructive nature
However, IR spectroscopy also has some limitations, such as:
- It can be difficult to interpret IR spectra, especially for complex molecules.
- IR spectroscopy is not always able to distinguish between different functional groups.
- IR spectroscopy is not suitable for analyzing samples that are not transparent to infrared radiation.
General Inquiries
What is the most prominent IR band in the spectrum of 2-methylcyclohexanol?
The most prominent IR band is observed at approximately 3300 cm-1, corresponding to the O-H stretching vibration of the hydroxyl group.
How can IR spectroscopy distinguish between 2-methylcyclohexanol and cyclohexanol?
The presence of a strong C-H stretching band at approximately 2960 cm-1 in the spectrum of 2-methylcyclohexanol, which is absent in cyclohexanol, indicates the presence of the methyl group.
What are the limitations of IR spectroscopy in analyzing 2-methylcyclohexanol?
IR spectroscopy may not be able to differentiate between certain functional groups with similar IR bands, and it can be challenging to interpret complex spectra with overlapping bands.